By Sarah Sydlowski
This article is a part of the September/October 2022, Volume 34, Number 5, Audiology Today issue.
Cochlear Implant Candidacy
Cochlear implantation is commonly viewed as a treatment of last resort. When benefit from hearing aids is limited or non-existent, a cochlear implant (CI) is commonly accepted as a reasonable treatment option. Between 1984 and 2015, a total of 170,252 U.S. adult and pediatric patients underwent cochlear implantation, accounting for 240,056 devices (iData Research, 2016; Nassiri et al, 2022). These patients represent only a fraction of the more than eight million annual hearing aid users in the United States (iData Research, 2016; Nassiri et al, 2021). There are substantially fewer implant recipients, but is this disproportionate uptake of CIs a reflection of clinical reality or is it indicative of an outdated system?
Cochlear implantation initially was approved in the middle of the 1980s by the Food and Drug Administration (FDA) for use in post-lingually deafened adults with profound bilateral sensorineural hearing loss (SNHL). At the time, this approved indication aligned well with the clinical evidence, which was based on recipients with little to lose by trying the initially experimental device. Bilateral profound hearing loss with no aided speech-recognition ability was deemed appropriate for candidacy.
Over time, ‘clinical candidacy’ and ‘coverage’ by a payer have become confusingly conflated.
As insurance payers noted the benefit of the first implant technology capable of restoring a human sense, most payers (including the Centers for Medicare and Medicaid Services (CMS)) adopted coverage plans that allowed patients with bilaterally profound hearing loss access to CI. As new implants were developed, manufacturers sought updated labeling indications, eventually expanding candidacy criteria to the parameters that many professionals cite as standard CI candidacy criteria: moderate to profound bilateral SNHL with aided sentence recognition in the ear to be implanted of less than 50 percent and less than 60 percent in the contralateral ear and when bilaterally aided (Cochlear, 2022). Frustratingly, the coverage determination adopted by CMS fell short of this clinical standard, requiring best aided open-set sentence recognition of less than 40 percent (CMS, 2005).
Over time, “clinical candidacy” and “coverage” by a payer have become confusingly conflated by referring providers who are unaware that advancements in technology and increasingly positive outcomes outpace both FDA labeling and many coverage policies. Contemporary clinical practices and outcomes are advancing at a rapid pace and FDA criteria and private insurance coverage are not expanding quickly enough to keep up (Zwolan and Basura, 2021; Moses and Friedmann, 2021).
Today, the misalignment among clinical candidacy, FDA labeling, and payer coverage policies confounds providers. Many providers base clinical decision-making on labeling and coverage instead of evidence-based clinical outcomes, prompting an unknown number of potential CI candidates to forgo candidacy evaluation or implantation that may be very appropriate.
The approximate U.S. CI use rate likely falls between 2 percent, using contemporary clinical candidacy criteria, and 13 percent, using traditional candidacy criteria (Nassiri et al, 2022; iData Research, 2016). Thus, although the annual proportion of CI recipients to new audiometric candidates has increased for patients with less severe degrees of hearing loss, the total population of untreated individuals who may benefit from CI continues to rise (Nassiri et al, 2022).
Case Scenario
A 61-year-old female has adult-onset, progressive sensorineural hearing loss attributed to autoimmune disease. Her audiogram (FIGURE 1) suggests moderately severe SNHL from 750–8000 Hz with poor word-recognition ability. She wears hearing aids in both ears, but reports that she is increasingly frustrated and has difficulty in a variety of situations. She is anxious in groups and crowds and is withdrawing from public-speaking engagements that have always been a key aspect of her work because she feels uncertain fielding questions from the crowd.
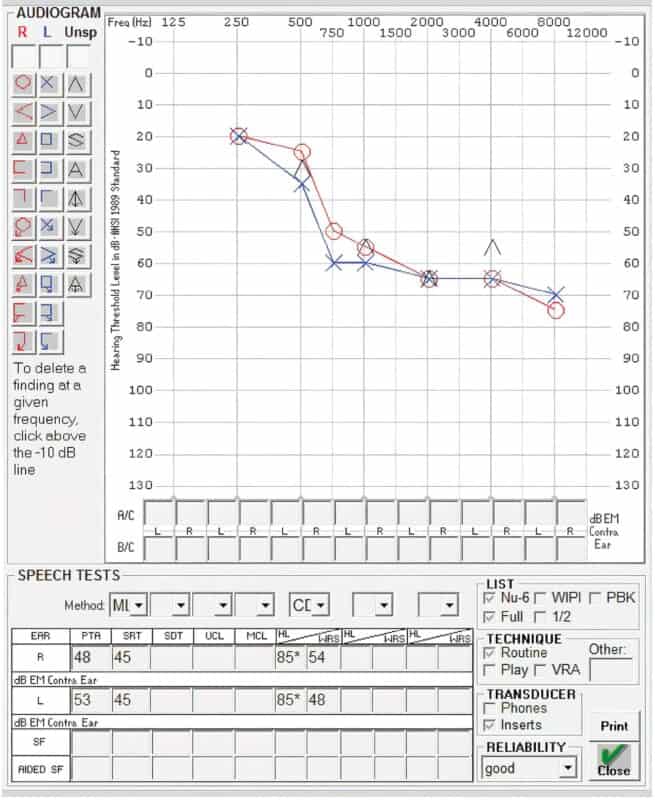
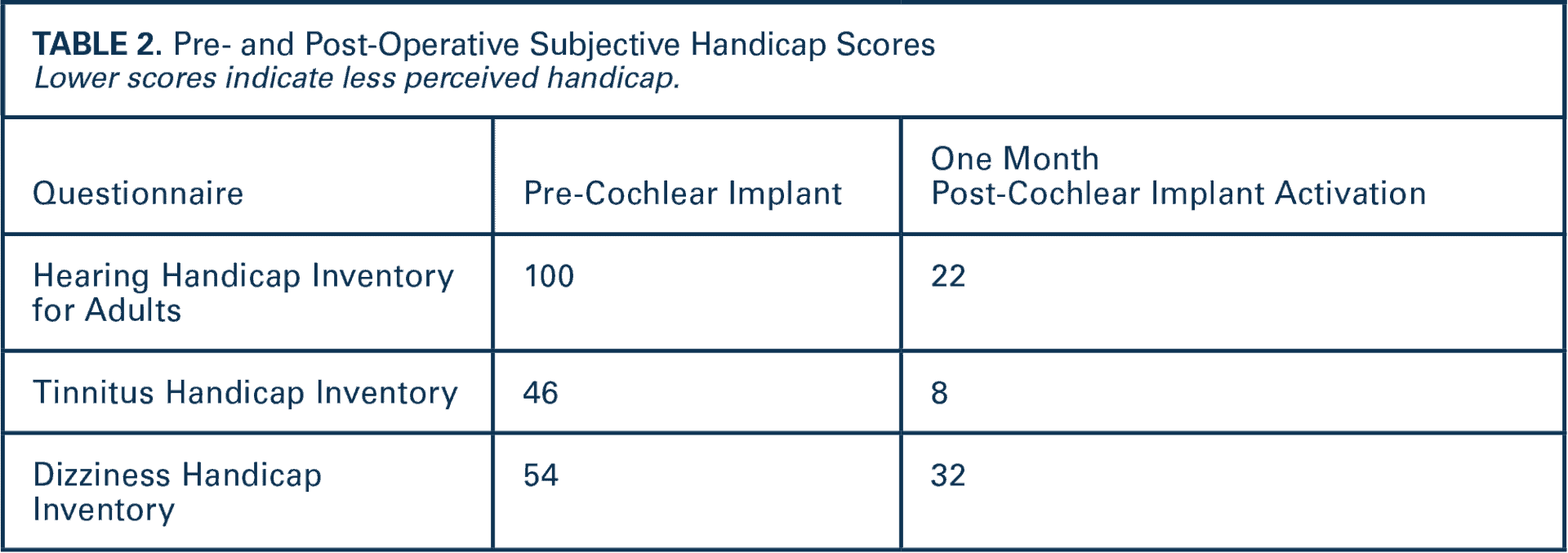
Hearing aid verification confirmed her hearing aids were programmed to National Acoustic Laboratories (NAL) targets and were reasonably fit. Her audiogram and excellent aided sentence recognition (TABLE 1) do not meet FDA-labeled indications for a CI. As a result, most audiologists would likely recommend hearing aids in this scenario. Yet, her handicap scores for both tinnitus and hearing suggest (TABLE 2) she perceives substantial difficulty. Further discussion reveals she has tried and returned numerous pairs of hearing aids.

Her aided word- and sentence-recognition in noise are poor. This patient is a prime example of why expanding CI candidacy is necessary.
CIs are a reliable and effective intervention for the majority of adults who have limited speech-perception abilities. On average, adults with CIs may achieve post-operative speech recognition of 54 percent for words, 74 percent for sentences, and 50 percent correct for speech in noise (Boisvert et al, 2020). Further, implantation of ears with less severe degrees of hearing loss and more measurable residual hearing also results in higher post-implant speech recognition and improved quality of life (Dowell, 2016; Birman and Sanli, 2020; Gifford et al, 2010). The expansion of candidacy criteria over the last decade has been most notable in three domains: (1) aided word instead of sentence recognition, (2) ear-specific instead of best-aided scores, and (3) increased degrees of residual hearing.
Words, Instead of Sentences, Identify the Best Candidates
Many of the largest and most experienced implant programs support the use of ear-specific word recognition, rather than audiometric thresholds or aided sentence recognition in the best aided condition (Deep et al, 2021; Gifford et al, 2010; Sladen et al, 2017; Sydlowski et al, 2022). In more contemporary regulatory reviews for short electrode arrays and unilateral hearing loss, manufacturers received labeling based on ear-specific, aided word understanding, but, in my experience, these contemporary criteria tend to be less well-known by referring providers and less commonly cited in payer-coverage policies. Specifically, they describe candidacy based on word understanding in the ear to be implanted of up to 60 percent and up to 80 percent in the contralateral ear. Residual hearing can be normal out to 1000 Hz, precipitously dropping to severe-profound high frequency SNHL.
CIs are a reliable and effective intervention.
Word recognition is preferable compared to sentences because sentences contain context and assess higher level processing skills (e.g., working memory). Conversely, word-recognition testing de-emphasizes processing skills and the use of context to enhance performance.
Ear-Specific Determination of Candidacy
The consideration of individual ears has also opened the door for recommending CIs in patients with varying degrees of hearing loss or normal hearing in the non-CI ear (Arndt et al, 2011; Galvin et al, 2019; Jakob et al, 2021; Firszt et al, 2018). Two manufacturers have approved labeling for single-sided deafness (SSD) and asymmetric hearing loss (AHL). However, both labels limit aided word-recognition scores to less than 5 percent in the ear to be implanted.
The benefit of CIs in patients with SSD and AHL may extend well beyond patients with profound hearing loss in the implanted ear (Sydlowski et al, 2022). Even when the contralateral ear has normal hearing sensitivity, there is well-documented evidence of consistently improved outcomes and high rates of long-term CI adoption and use by recipients (Deep et al, 2021).
Presence of Residual Natural Hearing
The presence of measurable residual hearing in an ear under evaluation for CI may concern patients and audiologists. However, improved electrode arrays and surgical techniques may allow for hearing preservation. Today, most CI candidates with residual hearing in the implanted ear (including even normal thresholds through 1000 Hz) have successful outcomes with CI (Gantz et al, 2016; Dunn et al, 2020), in part because of preserved low-frequency residual hearing and the possibility of electric-acoustic stimulation (Gantz et al, 2018). The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) defines functionally relevant residual hearing as a low-frequency pure-tone average (125, 250, 500 Hz) less than 80 dB HL (Adunka et al, 2018) and recommends ongoing evaluation and management of acoustic hearing in patients exhibiting responses at this level.
Improved electrode arrays and surgical techniques may allow for hearing preservation.
Clinical Candidacy, Not Coverage
CIs are effective among older adults (older than 65 years) and improve social isolation, depression, and cognitive performance (Castiglione et al, 2016; Gurgel et al, 2022). Yet, older adults who are Medicare Part B beneficiaries must currently wait longer and have more severe degrees of hearing loss before cochlear implantation will be covered (Bernhard, 2021; Schafer et al, 2021). Specifically, Medicare coverage requires that potential candidates score less than 40 percent on recorded open-set sentences in the best-aided condition, which is far more restrictive than both FDA labeling and clinical best practices.
Some private insurers continue to cite CMS guidelines for CI coverage, restricting access to cochlear implantation until there is no other viable option. Although most private payers allow for pre-authorization and approval outside of published coverage guidelines, Medicare Part B does not allow such a practice, limiting eligible recipients to those who meet their strict requirements for coverage and facilitating the confused understanding of providers that coverage is equivalent to candidacy. This limitation results in an access disadvantage for Medicare beneficiaries. Patients with military insurance were 13 times more likely to pursue surgery as compared to patients with Medicare (Tolisano et al, 2019).
Between 2014 and 2050, the Medicare population is projected to grow from 54 to 93 million beneficiaries (Congressional Budget Office, 2014; Neuman et al, 2015). The number of adults older than age 60 who are eligible for conventional CI is expected to double by 2060 to almost 4 million (Goman et al, 2018), making it imperative to align coverage policies with best clinical practices. Otherwise, the denominator (potential candidates) will substantially increase while the numerator (implant recipients) will remain fixed, a phenomenon that is already beginning (Nassiri et al, 2022).
In response to a request for reconsideration of the national coverage determination submitted by Zwolan and Buchman (2020), CMS is considering proposed expansion of coverage for implantation for adults with aided sentence-recognition scores of up to 60 percent (an increase from 40 percent). Several studies have specifically considered outcomes for patients with sentence-recognition scores greater than 40 percent and less than 60 percent, the results of which support implantation of candidates with greater degrees of pre-operative sentence-recognition ability than those currently covered under CMS guidelines (Mudery et al, 2017; Zhang and Coelho, 2018; Zwolan et al, 2020). Evidence in the literature and open public comments (CMS, 2022) is currently being reviewed and a proposed decision memo is due September 1, 2022.
It is time for coverage to catch up with clinical best practices.
Consequences of Expanding Candidacy Criteria and CI Under-Utilization
Expanding candidacy criteria and the reconsideration of factors that prevent CI access, including coverage determination policies, will increase access for patients who would benefit from CIs. Returning to the previously discussed case, this patient was recommended cochlear implantation based primarily on her poor aided word understanding. Her difficulty hearing in noise and significant self-reported handicap contributed to the recommendation.
Post-CI (TABLE 1), her aided speech recognition improved significantly, particularly for words in quiet and sentences in noise. Importantly, her bimodal performance (CI + hearing aid) exceeds her post-operative CI only and pre-operative bilateral hearing aid scores. Perhaps most notably, her perceived hearing and tinnitus handicaps were greatly reduced (TABLE 2) and she is once again participating in the activities she enjoys. Despite these excellent outcomes, it is likely that she would have been overlooked as a CI candidate (Nassiri et al, 2022) because she did not meet conventional candidacy criteria.
It is time for coverage to catch up with clinical best practices. At the very least, it is imperative that all providers of hearing care understand the differences between “clinical candidate” and “coverage” and refer for CI evaluation accordingly. When an estimated 87 to 98 percent of the individuals who can benefit from this technology are not receiving it (Nassiri et al, 2022; iData Research, 2016), it is time to ask why this is happening and to reverse the trend.
CIs are not a last resort. Using ear-specific word-recognition testing is more likely to identify appropriate candidates. Residual hearing is not a contraindication to CI and may actually improve the prognosis for benefit. When Medicare and other payers align their coverage policies to clinical best practices, more candidates will be able to access this life-changing technology. Until these principles are universally understood, expansion of CI candidacy falls short of reaching the patients who can benefit.
References
Adunka OF, Gantz BJ, Dunn C, Gurgel RK, Buchman CA. (2018) Minimum reporting standards for adult cochlear implantation. Otolaryngol Head Neck Surg 159(2):215–219.
Arndt S, Aschendorff A, Laszig R et al. (2011) Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol 32(1):39–47.
Bernhard N, Gauger U, Romo Ventura E et al. (2021) Duration of deafness impacts auditory performance after cochlear implantation: a meta-analysis. Laryngoscope 6(2):291–301.
Birman CS, Sanli H. (2020) Cochlear implant outcomes in patients with severe compared with profound hearing loss.
Otol Neurotol 41(4):e458–e463.
Boisvert I, Reis M, Au A, Cowan R, Dowell RC. (2020) Cochlear implantation outcomes in adults: a scoping review. PLOS ONE 15(5):e0232421.
Castiglione A, Benatti A, Velardita C et al. (2016) Aging, cognitive decline and hearing loss: effects of auditory rehabilitation and training with hearing aids and cochlear implants on cognitive function and depression among older adults. Audiol Neurotol 21 (Suppl 1):21–28.
Centers for Medicare and Medicaid Services (CMS). (2022) Cochlear implants: public comments (CAG-00107R). www.cms.gov/medicare-coverage-database/view/ncacal-public-comments.aspx?ncaid=306 (accessed June 21, 2022).
Centers for Medicare and Medicaid Services (CMS). (2005) National coverage determination (NCD), Cochlear implantation. www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=245&ncdver=2 (accessed June 21, 2022).
Congressional Budget Office. (2014) The 2014 Long-Term Budget Outlook. www.cbo.gov/publication/45471 (accessed August 4, 2022).
Cochlear. (2022) Cochlear Implant Candidacy Criteria. www.cochlear.com/us/en/professionals/products-and-candidacy/candidacy/cochlear-implant (accessed August 16, 2022).
Deep NL, Spitzer ER, Shapiro WH, Waltzman SB, Roland JT, Friedman DR. (2021) Cochlear implantation in adults with single-sided deafness: outcomes and device use. Otol Neurotol 42:414–423.
Dowell RC. (2016) The case for earlier cochlear implantation in postlingually deaf adults. Int J Audiol 55(Suppl 2):S51–S56.
Dunn CC, Oleson J, Parkinson A, Hansen MR, Gantz BJ. (2020) Nucleus Hybrid S12: multicenter clinical trial results. Laryngoscope 130(10):E548–E558.
Firszt JB, Reeder RM, Holden LK, Dwyer N, Asymmetric Hearing Study Team. (2018) Results in adult cochlear implant recipients with varied asymmetric hearing: a prospective longitudinal study of speech recognition, localization, and participant report. Ear Hear 39(5):845–862.
Galvin JJ, Fu QJ, Wilkinson EP et al. (2019) Benefits of cochlear implantation for single-sided deafness: data from the House Clinic-University of Southern California-University of California, Los Angeles Clinical Trial. Ear Hear 40(4):766–81.
Gantz BJ, Dunn CC, Oleson J, Hansen MR. (2018) Acoustic plus electric speech processing: long-term results. Laryngoscope 128(2):473–81.
Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. (2016) Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: final outcomes. Laryngoscope 126(4):962–73.
Gifford RH, Dorman MF, Shallop JK, Sydlowski SA. (2010) Evidence for the expansion of adult cochlear implant candidacy. Ear Hear 31:186–194.
Goman AM, Dunn CC, Gantz BJ, Lin FR. (2018) Prevalence of potential hybrid and conventional cochlear implant candidates based on audiometric profile. Otol Neurotol 39(4):515–17.
Gurgel RK, Duff K, Foster NL, Urano KA, deTorres A. (2022) Evaluating the impact of cochlear implantation on cognitive function in older adults. Laryngoscope 132(Suppl 7):S1–S15.
iData Research. (2010) U.S. Market for Hearing Aids and Audiology Devices. Burnaby, British Columbia, Canada.
iData Research. (2016) U.S. Market Report Suite for Hearing Devices. Burnaby, British Columbia, Canada.
Jakob TF, Speck I, Rauch AK et al. (2021) Bone-anchored hearing system, contralateral routing of signals hearing aid or cochlear implant: what is best in single-sided deafness? Eur Arch Otorhinolaryngol 279(1):149–158.
Moses LE, Friedmann DR. (2021) Cochlear implant indications: a review of third-party payers; policies for standard and expanded indications. Cochlear Implants Int 22(4):237–44.
Mudery JA, Francis R, McCrary H, Jacob A. (2017) Older individuals meeting Medicare cochlear implant candidacy criteria in noise but not in quiet: are these patients improved by surgery? Otol Neurotol 38(2):187–191.
Nassiri AM, Ricketts TA, Carlson ML. (2021). Current estimate of hearing aid usage in the United States. Otol Neurotol 1:e001.
Nassiri AM, Sorkin DL, Carlson ML. (2022) Current estimates of cochlear implant utilization in the United States. Otol Neurotol 43(5):e558–e562.
Neuman K, Cubanski, J, Huang J, Damico A. (2015) The rising cost of living longer: analysis of Medicare spending by age for beneficiaries in traditional Medicare. www.kff.org/medicare/report/the-rising-cost-of-living-longer-analysis-of-medicare-spending-by-age-for-beneficiaries-in-traditional-medicare/view/footnotes/#:~:text=Between%202014%20and%202050%2C%20the,%3A%2F%2Fwww.cbo.gov%2F (accessed June 21, 2022).
Schafer EC, Miller S, Manning J et al. (2021) Meta-analysis of speech recognition outcomes in younger and older adults with cochlear implants. Am J Audiol 30(3):481–96.
Sladen DP, Gifford RH, Haynes D et al. (2017) Evaluation of a revised indication for determining adult cochlear implant candidacy. Otol Neurotol 127(10):2368–2374.
Sydlowski SA, Farrokhian N, Carrozza M, Jamis C, Woodson E. (Forthcoming) (Even off-label) cochlear implantation in SSD and AHL results in measurable objective and subjective benefit. Otol Neurotol.
Tolisano AM, Schauwecker N, Baumgart B et al. (2019) Identifying disadvantaged groups for cochlear implantation: demographics from a large cochlear implant program. Ann Otol Rhinol Laryngol 129(4):347–354.
Zhang E, Coelho DH. (2018) Beyond sentence recognition in quiet for older adults: implications for cochlear implant candidacy. Otol Neurotol 39(8):979–86.
Zwolan T, Buchman, C. (2020) Formal request for NCD reconsideration. www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id306.pdf (accessed June 21, 2022).
Zwolan TA, Basura G. (2021) Determining cochlear implant candidacy in adults: limitations, expansions, and opportunities for improvement. Semin Hear 42(4):331–41.
Zwolan TA, Kallogjeri D, Firszt JB, Buchman CA. (2020) Assessment of cochlear implants for adult Medicare beneficiaries aged 65 years or older who meet expanded indications of open-set sentence recognition: a multicenter nonrandomized clinical trial. JAMA Otolaryngol 146(10):933–941.