By Angela Shoup and Maggie Kettler
This article is a part of the January/February 2023, Volume 35, Number 1, Audiology Today issue.
Many years ago, as a new audiologist and before the advent of mandated universal newborn hearing screening (UNHS), I (A.S.) had, as one of my first patients, an infant with sloping, high-frequency sensorineural hearing loss. In reviewing his birth history and talking with his mother, I learned that he was born with congenital cytomegalovirus (cCMV). His mother had worked in childcare while pregnant where she likely contracted CMV. She had read a lot about cCMV and was grateful that hearing loss was the only presenting symptom for her son. Although cCMV was known to cause hearing loss at that time, there has been increased attention to the impact of cCMV in recent years as well as options for prevention, screening, and treatment. Much of the advocacy for increasing education and early identification of cCMV has been driven by parents of children born with cCMV, as evidenced by groups such as the National CMV Foundation. Audiologists are key members of interprofessional teams supporting children and families impacted by cCMV and have become critical contributors to screening and advocacy efforts.
Audiologists are key members of interprofessional teams supporting children and families impacted by cCMV and have become critical contributors to screening advocacy efforts.
What Is Congenital Cytomegalovirus?
Cytomegalovirus is a highly transmissible viral infection that is typically harmless, and healthy individuals who contract CMV may have no symptoms or may experience mild flu-like symptoms (Rawlinson et al, 2017). Many do not know they have had CMV unless tested (Rawlinson et al, 2017). Children who are infected with CMV may shed virus for months or years and during this time are contagious. Infected adults may excrete virus only up to six months. The virus may be dormant for an extended period in a person who has been infected with CMV, during which time the individual is not infectious. However, the virus can become reactivated, or the CMV-positive person may become reinfected by a different strain of CMV (Rawlinson et al, 2017; Boucoiran et al, 2021). Seroprevalence, or the percentage of people who test positive for CMV based on serology (blood) specimens, is estimated to be around 83 percent globally (Zuhair et al, 2019), with a range from 45 percent to 100 percent (Cannon et al, 2010). The seroprevalence in a specific location or population is dependent on several factors, including gender, socioeconomic status, age, and race (Cannon et al, 2010).
Individuals can become infected with CMV through exposure to bodily fluids, including saliva, blood, breast milk, and urine. The virus may be transmitted by intimate contact, kissing, sharing food or eating utensils, changing diapers, and other general daily activities that may bring a person in contact with bodily fluids. Because infants and children who are positive for CMV excrete the virus for months to years, young mothers and childcare workers or others who are around young children are at increased risk for exposure. Standard infectious control procedures are very effective in avoiding contraction of CMV, but unfortunately, 61–87.5 percent of women do not know about the risk of cCMV to the developing fetus during pregnancy (Rawlinson et al, 2017).
Although cCMV is the most common viral infection in utero, most infants born with cCMV will be asymptomatic.
Maternally active CMV infection can be vertically transmitted, or passed from mother to baby in utero, and can have significant impact on the developing fetus. Primary infections occur when a mother becomes infected with CMV for the first time during pregnancy. If latent CMV is reactivated or the mother who had previously had CMV is exposed to another strain, it is referred to as secondary infection. Mothers with active CMV infection during pregnancy, either primary or secondary, can pass CMV to the fetus, who may contract cCMV. Typically, cCMV is more transmissable in infants born to mothers with primary infection, but there are many cases of infants with presenting symptoms of cCMV being born to mothers with secondary infection (Bonalumi et al, 2011).
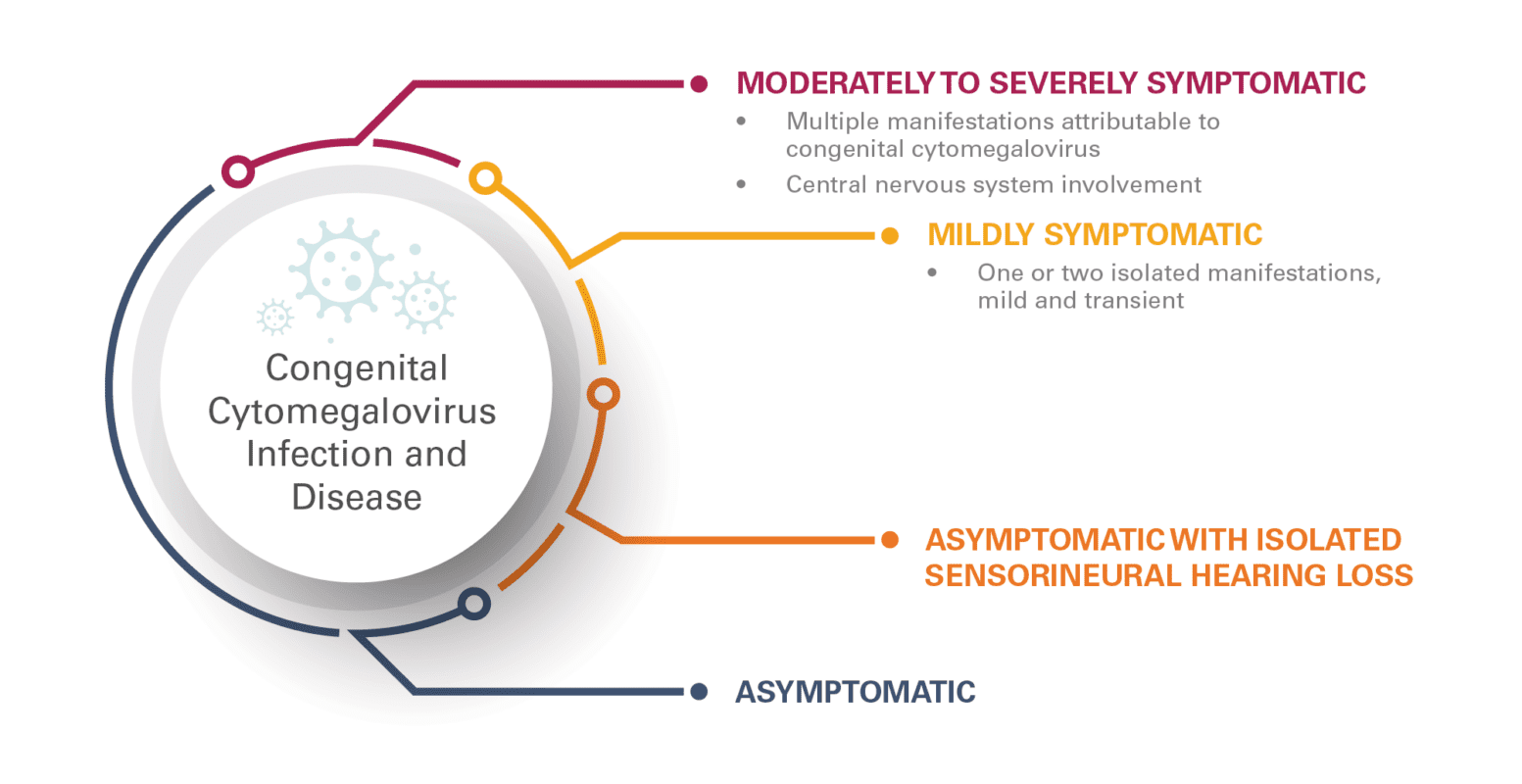
Although cCMV is the most common viral infection in utero, most infants born with cCMV will be asymptomatic (85–90 percent) (Rawlinson et al, 2017). Presentation of cCMV ranges from asymptomatic to moderately to severely symptomatic (FIGURE 1). The 10–15 percent of those who are born with presenting symptoms of cCMV may exhibit microcephaly, hepatomegaly, splenomegaly, hepatitis, seizures, optic atrophy, sensorineural hearing loss, intrauterine growth restriction, petechiae, thrombocytopenia, jaundice, anemia, and chorioretinitis. Of those with severe symptoms, approximately 20–30 percent will not survive. Of the 10–15 percent of infants with cCMV who are born asymptomatic, approximately 5–15 percent will experience delayed psychomotor development, delayed-onset sensorineural hearing loss, or delayed-onset visual impairment (Bonalumi et al, 2011; Rawlinson et al, 2017).
Déjà vu: To Screen or Not to Screen
Given cCMV is the greatest nongenetic risk factor for sensorineural hearing loss and is a major cause of birth defects, there has been increased focus on options for early identification of cCMV with the goal of providing early intervention and support (Diener et al, 2017; Goderis et al, 2016). The discussions around screening for cCMV are reminiscent of the debates about UNHS.
Many audiologists are aware that Marion Downs was a strong proponent of newborn hearing screening as early as the 1960s. However, the need for a mechanism of identifying hearing loss in the newborn period was recognized as early as 1944 (Ruben, 2021). In part, due to the lack of appropriate tools for mass screening of infants, early identification of hearing loss initially rested on targeted screening of those at “high-risk” of congenital hearing loss (Joint Commission on Infant Hearing, 1973). UNHS was recommended in the 1994 Joint Committee on Infant Hearing position statement and was rapidly adopted in the United States by the late 1990s. In order to obtain widespread acceptance of the need for UNHS, a number of issues had to be resolved, including whether congenital hearing loss had enough prevalence to warrant screening, whether there were cost-effective tools with sufficient sensitivity and specificity for identifying hearing loss in infants, whether there were effective early intervention options that were developmentally impactful, and whether earlier intervention was proven to be more effective than later intervention.
At the National Institutes of Health Consensus Development Conference on Early Identification of Hearing Impairment held in 1993, it was recommended that all infants receive hearing screening by three months of age (National Institutes of Health, 1993). This recommendation was based, in part, on the acknowledgment that high-risk register screening would miss approximately 50–70 percent of infants with significant hearing loss. Without screening, many infants would not be identified with hearing loss until around three years of age, and with auditory brainstem response and otoacoustic emission screenings, there were now sensitive and specific tools for early identification of hearing loss that were applicable to mass screening of newborns. The work of Yoshinaga-Itano et al (1998) reinforced the importance of early intervention in infants with hearing loss.
Prevalence of cCMV has been reported to range from 0.2 percent to 2 percent of pregnancies (Rawlinson et al, 2017). Further, cCMV is the most significant infectious cause of congenital and delayed-onset sensorineural hearing loss and of many other neurodevelopmental and physical morbidities. Just as with UNHS, lack of sensitive and specific tools applicable to cost-effective mass screening has been a reported factor in limiting early identification of all infants with cCMV. Specimens for cCMV screening must be obtained within the first 21 days of life to provide accurate differentiation between cCMV and acquired CMV. Current options include use of saliva, urine, or dried blood spot polymerase chain reaction for screening.
Like UNHS, cCMV screening started with risk-targeted approaches at a few hospitals, which were then adopted by other facilities. At some birthing hospitals, infants receive a CMV screen if they have overt presentation of symptoms associated with cCMV. Some facilities will also screen all infants for cCMV in the neonatal intensive care unit and specific at-risk populations, such as infants born to HIV-positive mothers (Duryea et al, 2010).
Because of the risk of sensorineural hearing loss associated with cCMV and the diagnostic importance of knowledge of cCMV status, hearing-targeted cCMV (HT-cCMV) screening has been incorporated into some hospital and state-wide UNHS programs. This approach can help identify those who do not pass UNHS and are positive for cCMV as well as those with mild clinical signs and symptoms that were not identified through the physical evaluation (Stehel et al, 2008). The model for HT-cCMV screening programs varies, but the general goal is the same—to complete cCMV testing in infants who do not pass UNHS. At Parkland Memorial Hospital in Dallas, Texas, where HT-cCMV screening has been part of the UNHS program since 1999, inpatient cCMV screening is completed in infants before discharge from the hospital if they do not pass the inpatient hearing screen (FIGURE 2). In 2013, Utah became the first state to require HT-cCMV screening, completed after the infant refers on the outpatient hearing rescreen within 21 days after birth (Diener et al, 2017). Other states have followed suit, including Connecticut, Iowa, New York, and Virginia. Although Illinois does not require HT-cCMV screening, the state does require that parents be offered cCMV screening if their infant does not pass UNHS. The Canadian provinces of British Columbia and Manitoba also have targeted cCMV screening programs.
Research indicates that 43 percent of infants ultimately impacted by cCMV-related hearing loss, including those at risk for delayed onset of hearing loss, will be missed with HT-cCMV screening (Fowler et al, 2017). In the consensus statement on prevention, diagnosis, and therapy for cCMV, Rawlinson et al (2017) stated that universal neonatal CMV screening should be considered to facilitate early detection of cCMV in infants, which would allow for early intervention for sensorineural hearing loss and developmental delay.
As the debate continues, the Canadian provinces of Ontario and Saskatchewan have implemented universal cCMV screening, and Minnesota will soon be the first U.S. state to enact universal cCMV screening.
What Is the Role of the Audiologist?
As cCMV is associated with potential congenital hearing loss that is progressive in approximately 54 percent of cases (Dahle et al, 2000), as well as delayed onset of hearing loss, the audiologist is key to accurately diagnosing hearing loss and providing prompt and appropriate early intervention. Infants with cCMV who have hearing loss at birth will fall under 2019 Joint Committee on Infant Hearing recommendations for early intervention. Due to the propensity of progressive hearing loss in infants with cCMV, hearing status will have to be carefully monitored to allow for adjustments to the child’s early intervention program. Audiologists also should be involved in monitoring vestibular function, as congenital or delayed-onset vestibular dysfunction can occur in infants with cCMV, with or without hearing loss (Dhondt et al, 2021).
Antiviral treatment with ganciclovir and valganciclovir has proven effective in infants with moderate to severe cCMV symptoms (Rawlinson et al, 2017), and audiologists should be part of the care team in monitoring auditory status during antiviral treatment. Studies have shown that ganciclovir or valganciclovir can reduce hearing loss or stabilize hearing thresholds and potentially improve neurodevelopmental outcomes in infants with cCMV when treated within the first four weeks of life (Kimberlin et al, 2015). Although not recommended at present, some physicians are also providing antiviral treatment for infants with cCMV who have sensorineural hearing loss only.
Due to the prevalence of delayed onset of hearing loss, the 2019 Joint Committee on Infant Hearing position statement promulgates surveillance of infants and children who are CMV positive at birth with the goal of promptly identifying delayed onset of hearing loss, with monitoring initiated at no later than three months of age. If delayed-onset hearing loss is going to occur, typically, it will begin to develop before five years of age (Lanzieri et al, 2017). After age five, children with cCMV are at no greater risk for delayed onset of hearing loss than their typically developing peers. However, if a child does exhibit hearing loss, it may continue to progress into the teen years (Lanzieri et al, 2017).
Educate and Advocate
Another important role the audiologist must take on is advocacy for our patients. Many health-care professionals have limited knowledge regarding cCMV, and many patients with cCMV go undiagnosed. As the leaders in hearing and balance, audiologists must be knowledgeable about prevention, diagnosis, and treatment for cCMV. There is a need for support from individual clinicians as well as a unified voice from professional organizations in support of cCMV education and testing. The American Academy of Audiology convened a task force to develop a position statement supporting early identification of cCMV in infants to allow evidence-based hearing surveillance and early intervention for those with congenital hearing loss and delayed onset of hearing loss. Early screening, diagnosis, and treatment of cCMV will improve the care of patients with cCMV and is the first step in alleviating the developmental impact of cCMV.
Resource
- Congenital Cytomegalovirus (CMV) Infection consumer-friendly page
References
Bonalumi S, Trapanese A, Santamaria A, D’Emidio L, Mobili L. (2011) Cytomegalovirus infection in pregnancy: review of the literature. J Prenat Med 5(1):1–8.
Boucoiran I, Yudin M, Poliquin V, Caddy S, Gantt S, Castillo E. (2021) Guideline No. 420: Cytomegalovirus infection in pregnancy. J Obstet Gynaecol Can 43(7):893–908.
Cannon MJ, Schmid DS, Hyde TB. (2010) Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20(4):202–213.
Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. (2000) Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Amer Acad Audiol 11(5):283–290.
Dhondt C, Maes L, Rombaut L et al. (2021) Vestibular function in children with a congenital cytomegalovirus infection: 3 years of follow-up. Ear Hear 42(1):76–86.
Diener ML, Zick CD, McVicar SB, Boettger J, Park AH. (2017) Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics 139(2):e20160789.
Duryea EL, Sanchez PJ, Sheffield JS et al. (2010) Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J 29(10):915–918.
Fowler KB, McCollister FP, Sabo DL et al. (2017) A targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics 139(2):e20162128.
Goderis J, Keymeulen A, Smets K et al. (2016) Hearing in children with cytomegalovirus infection: results of a longitudinal study. J Pediatr 172:110–115.e2.
Joint Committee on Infant Hearing. (1973) Screening for infant hearing. www.jcih.org/JCIH1973.pdf (accessed October 14, 2022).
Joint Committee on Infant Hearing. (1994) 1994 Position statement. www.jcih.org/JCIH1994.pdf (accessed October 14, 2022).
Joint Committee on Infant Hearing. (2019) Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv 4(2):1–44.
Kimberlin DW, Jester PM, Sanchez PJ et al. (2015) Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 372(10):933–943.
Lanzieri TM, Chung W, Flores M et al. (2017) Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics 139(3):e20162610.
National Institutes of Health. (1993) Early identification of hearing impairment in infants and young children. NIH Consensus Statement Online 11(1):1–24. https://consensus.nih.gov/1993/1993hearinginfantschildren092html.htm (accessed October 14, 2022).
Rawlinson WD, Bopanna SB, Fowler KB et al. (2017) Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 17(6):e177–e188.
Ruben RJ. (2021) The history of pediatric and adult hearing screening. Laryngoscope 131(Suppl 6):S1–S25.
Stehel EK, Shoup AG, Owen KE et al. (2008) Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics 121(5):970–975.
Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. (1998) Language of early- and later-identified children with hearing loss. Pediatrics 102(5):1161–1171.
Zuhair M, Smit GSA, Wallis G et al. (2019) Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol 29(3):e2034.