Legislation
The Over the Counter Hearing Aid Act of 2017 was passed as part of the 115th United States Congress as a rider on the FDA Reauthorization Act of 2017 (FDAR). This law requires the Food and Drug Administration (FDA) to propose a rule that would establish an OTC hearing aid category for adults with “perceived” mild to moderate hearing loss within 3 years of passage of the legislation and finalize a rule within 180 days after the close of the comment period. The provisions of the OTC Hearing Aid Act implement major recommendations from the President’s Council of Advisors on Science and Technology (PCAST) and the National Academies of Science, Engineering, and Medicine (NASEM).
Consensus Document
In 2018, The Academy also teamed up with other members of the hearing health community to draft consensus recommendations for this new class of devices. The consensus recommendations address: 1) the product requirements appropriate for OTC hearing devices targeting mild to moderate hearing impairment; 2) outside-of-the-box labeling appropriate for medical devices sold over-the-counter; 3) comprehensive inside-the-box labeling; 4) naming the products Self-Fit Over-the-Counter Hearing Devices, adopting risk classifications consistent with air conduction hearing aids, and limiting 510(k) exemptions; and 5) establishing strong consumer protection laws.
Regulations
The FDA released the proposed rule for regulation of OTC hearing aids on October 20, 2021.
The FDA provides 90 days for public comment with a deadline of 1/18/2022. Individuals can submit comments, in addition to organizations and other entities. The link to submit the comments and to access other submitted comments is available with the proposed rule.
The Academy formed a subject matter expert work group of members to analyze the proposed rule and prepare draft comments. The Academy board reviewed and approved final comments, and the Academy submitted the comments to the FDA on December 8, 2021. The FDA has to review and respond to all received comments, so the final rule likely will not be out before late spring.
Reach the Media
Educate the public on over-the-counter (OTC) hearing aids and the importance of an audiologist during their journey. Send out a customized press release to the media.
Over-the-Counter Hearing Aids FAQs for Audiologists
Basic Definitions
Over-the-counter hearing aids (OTC HAs) are devices that amplify sound and can be purchased without professional support and without a hearing test. OTC HAs are approved for adults older than age 18 with mild to moderate hearing loss.
Direct-to-consumer devices are currently available as instruments that can be purchased by the consumer with access to remote support. OTC HAs are similar to “direct-to-consumer” devices in that consumers independently decide if they need the device and select what device(s) to purchase. The consumer handles the device set-up, including the physical fit of the device and tuning of the sound, without professional support. Although not required, any consumer can get a hearing test before buying OTC HAs to benefit from the advice of an audiologist regarding whether they are a candidate for an OTC HA.
Audiologists also can provide support in the use of OTC HAs for an office visit charge (typically not covered by insurance).
OTC HAs are meant to be less expensive than professionally fitted hearing aids. The lower cost for OTC HAs is due to technology differences and because no professional services or fees are associated with the purchase (the individual is self-selecting and self-fitting).
Yes, the rule goes into effect on October 17, 2022. The final rule can be found at Federal Register: Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids.
The Academy’s e-learning platform, www.eaudiology.org, will be providing continuing education options now that the FDA final rule is published and as OTC HAs enter the marketplace.
The FDA Reauthorization Act of 2017 includes language that directed the FDA to create a class of OTC HAs in response to demands of consumers and other government agencies to increase the affordability and accessibility of hearing aids in the United States.
The FDA has finalized the regulations that surround this new class of amplification devices. These rules include (1) how loud these devices can be, (2) what labeling is required on the outside and inside of the box that these devices come in, and (3) requirements related to the sale of these devices.
The FDA is now using two descriptors for hearing aids: OTC hearing aids and prescription hearing aids.
The hearing aids that audiologists traditionally fit are considered prescription hearing aids meaning that they require a professional to determine the need for and the optimal settings of the hearing aid. The Final Rule regarding OTC HAs provides rules specific to OTC HAs, not prescription hearing aids.
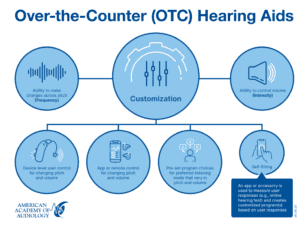
The FDA considers OTC HAs user adjustable. At a minimum, OTC HAs must have a way for the user to manipulate frequency response (e.g., bass versus treble) and intensity (volume control). The figure below illustrates ways that this might be accomplished. Self-fitting is one approach and is defined specifically as a process where user responses are used by an algorithm to create a listening program(s) for the user. This might be accomplished with an online hearing test or some other signals the individual responds to and then the device tunes itself related to these responses. All OTC HAs must be user-customizable for frequency response and intensity, but self-fitting is not required.
OTC HAs will have an output limit of 111 dB SPL with 117 dB SPL allowable for devices while input-controlled compression is activated. This will be expressed in terms of maximum peak values which are consistent with Output Sound Pressure Level 90 (OSPL90) values. These limits are different from Prescription Hearing Aids where an audiologist measures the maximum output of the hearing aids as part of the fitting process. No gain limit is being recommended or enforced for OTC HAs. The FDA is using the ANSI/CTA 2051 “Personal Sound Amplification Performance Criteria” as the electroacoustic standard for OTC HAs.
OTC HAs are only approved for use by adults older than age 18 who have mild to moderate hearing loss. Ideally, an individual will receive a hearing test from an audiologist to determine their level of hearing loss so they will know if they are a candidate for this type of hearing aid. However, a hearing test is not required, and an individual with perceived mild to moderate hearing loss may purchase OTC HAs directly.
Although an individual cannot accurately determine whether they have hearing loss, the type or degree of hearing loss if present, or the potential cause of hearing difficulties without professional evaluation, there are some behavioral indicators that may suggest the individual could benefit from hearing assistance. If the individual does not want to get a hearing test, they may consider the following questions as they try to determine if they have a mild to moderate hearing loss and/or whether they should consider an OTC:
- Are they able to hear easily in quiet, one-on-one situations but experience difficulty in more challenging listening conditions?
- Are there a few difficult listening situations where they think they would want to wear the OTC HAs as opposed to feeling like they would need it in most communication situations?
- Does turning up the volume on the phone or TV just slightly help them hear better (this level might be considered a little loud by others but not extremely loud as opposed to needing to turn these devices up quite a bit to a level that bothers others)?
If the individual answered yes to these questions, they may have mild to moderate hearing loss. If the individual experiences more significant hearing and/or communication challenges in several environments, they may have more than a mild to moderate hearing loss and may not benefit from an OTC. People with moderately severe to severe hearing loss will have difficulty in these situations as well but they will have difficulty more consistently across many situations as identified by themselves or by those around them.
OTC HAs will require that the user follow instructions to fit the device to their ear. The user will need to follow instructions in terms of how to tune the sound of the device. This could include an automated hearing test that requires responses to sounds. This also could include manipulating a volume control or an app-based program that has the user change the bass and treble to find a sound combination that is perceived to be helpful.
No. OTC HAs are only approved for individuals older than age 18. Due to the medical nature of childhood hearing loss and the importance of accurate sound delivery for the developing brain, OTC HAs are not appropriate for children. Learn more under this section: Children and Over-the-Counter Hearing Aids
Audiologists serve as a vital member of the health-care team in treating children with hearing loss to ensure the best outcomes.
Once the FDA regulations are finalized, OTC hearing aids will likely be advertised and sold in local drug stores, big-box stores, online, via mail, and even in an audiologist’s office.
Manufacturers and distributors will set the price of any device.
Incorporating Over-the-Counter Hearing Aids Into Your Audiology Practices
Audiologists may choose not to provide OTC devices. However, many may choose to offer OTC devices directly or to provide professional support services to individuals who have purchased OTC devices elsewhere.
OTC hearing aids can be incorporated into your clinical practice as an additional amplification option for patients with mild to moderate hearing loss. Your role with OTC hearing aids would be to ensure that the fit and “programming” of the OTC HA is appropriate and optimal, within the limits of the technology, as well as to educate and guide the patient relative to appropriate expectations and care/use of the instrument.
This model is of benefit to the patient if an OTC HA does not meet a patient’s communication needs, or is no longer appropriate for a patient, the audiologist can recommend an amplification option that is more appropriate. This would be part of a continuum of care focused on a patient-centered hearing treatment plan. Each practice will need to determine what role the audiologist plays in this activity and/or what role is appropriate for an audiology assistant. These decisions would be made in the context of the state laws under which the audiologist practices.
Alternatively, you may choose not to dispense OTC HAs but still elect to support patients who have purchased OTC HAs and are looking for assistance. Again, each practice will have to consider if this support is provided by an audiologist or an audiology assistant. The patient should be made aware of the fees associated with these services.
Members of the American Academy of Audiology can access a variety of recommendations and resources to help in learning more about this process.
- Navigating Your Practice Now that Mainstream Over-the-Counter Hearing Aids Are Coming
- Embracing Change in Audiological Treatment: Over-the-Counter Hearing Devices
- Position Statement: The Role of Audiologists with Over-the-Counter Hearing Aids
- The Audiologist’s Guide to Hearing Aids, PSAPs, Hearables, and OTC Devices
Any services that are part of a comprehensive hearing habilitation plan to enhance performance and outcomes with traditional (prescriptive) amplification options may be of benefit to the OTC HA user.
Depending on need, you may offer
- Diagnostic evaluation to confirm candidacy or to obtain necessary data to improve setting and selection of adjustable features, as available.
- Adjustment of settings and features, if available on the device, with real-ear measurement to verify the impact of the device settings on access to the speech spectrum.
- In-office cleaning and repair as well as guidance on the use and care of the device.
- Electroacoustic analysis may be necessary to confirm the functionality and quality of sound.
- Supportive counseling.
You will want to consider the level of expertise needed to assist individuals with OTC HAs. The patient may see you, the audiologist, for diagnostic evaluation, but an assistant may be able to support the OTC HA fitting (e.g., instruction in insertion, removal, and care).
If a patient who already owns an OTC HA schedules to come in for assistance, they may be scheduled with an audiology assistant. Depending on the patient’s needs, the assistant may resolve the issue or the assistant may determine that the patient needs a hearing evaluation or measurement of the output of the devices. These services would be provided by you, the audiologist.
Professional fees should apply for services provided to support OTC HA use. The Academy’s Guide to Itemizing Your Professional Services is a good resource for practices that are not already unbundling services and products.
Update your practice website with information about OTC HAs. This should include information about whether the practice will sell OTC HAs and/or offer services to patients who have purchased them elsewhere.
Review the pricing/payment structure relative to OTC HAs and professional services, as well as the protocol within the office for evaluating patients for OTC HAs. For example, will the practice recommend a hearing evaluation? Who will see patients with OTC HAs (the audiologist or assistant), etc.?
If you decide to engage in treatment that includes OTC devices, you will want to review your billing practices and ensure you have unbundled options for charging for services that support improved outcomes with device fitting, as discussed in The Audiologist’s Guide to Hearing Aids, PSAPs, Hearables, and OTC Devices.
Make sure to review the laws in your state that govern your license to make sure you are practicing as expected when working with hearing aids. Until state laws are updated to differentiate between OTC hearing aids and prescription hearing aids, you will need guidance as to how to apply the rules that are written for “hearing aids” related to the practice of audiology.
In a recent study that mimicked the direct-to-consumer model, many individuals still required assistance in the selection and use of the devices1. Offering and supporting OTC HAs with professional services allows you, the audiologist, to demonstrate the benefits of audiologists as hearing health-care professionals, set realistic expectations with the consumer about what to expect from OTC HAs, and establish rapport with patients such that if OTC HAs are not successful or no longer an appropriate option, the patient can proceed with prescriptive devices and rehabilitative services as needed.
Reference
1Humes LE, Rogers SE, Quigley TM, Main AK, Kinney DL, Herring C. (2017) The effects of service-delivery model and purchase price on hearing-aid outcomes in older adults: A randomized double-blind placebo-controlled clinical trial. Amer J Audiol.
Check out the Academy’s Guide to Itemizing Your Professional Services.
This document discusses a step-by-step process through which itemized fee schedules can be constructed, as well as examples of itemized billing.
Coverage for OTC HAs will depend on the payer and their respective policies. We have observed that some private payers already have clauses in their coverage determinations to include OTC HAs and others exclude them. If policies are identified to cover OTC HAs, audiologists are encouraged to contact Provider Relations departments to identify coverage policies and other requirements under a specific plan. The Academy will be reviewing and monitoring coverage policies and updating membership accordingly. Inquiries may be directed to reimbursement@audiology.org.
OTC HAs can be available any time after October 17, 2022. Once there are devices on the market, you’ll want to gather information on different options to decide what might work in your practice.
The Academy will be looking for reports on the response and sound quality of these devices as they come out as well as reports related to ease of use by consumers. Remember, you are equipped to measure the output of any device that makes sound by using your electroacoustic analysis or real ear probe microphone measurements to inform yourself regarding the capabilities of these devices.
We would expect there to be online reviews once OTC HAs are available that will be helpful as these products are used by others and they report on their experience.
You may want to consider providing this type of information on your website in case a patient lands on your page searching for OTC HA advice.
“If your hearing loss is greater than mild to moderate or you have any of the symptoms listed below, you should not purchase an OTC hearing aid without first seeing an audiologist or ear, nose, and throat (ENT) physician:
- Malformed or misshapen ear at birth or due to trauma
- History of drainage from the ear within the previous 90 days
- History of sudden or rapidly progressive hearing loss within the previous 90 days
- Dizziness that you have just experienced or that you have experienced over a long time
- Hearing loss in only one ear or sudden or recent onset of hearing loss within the previous 90 days
- Significant ear wax accumulation or a foreign body in the ear canal
- Pain or discomfort in the ear
You should also seek medical advice if any of these symptoms occur after using an OTC HA."
Studies show that the average person waits for approximately 7 years1 after the onset of symptoms before seeking professional hearing help. An increasing number of studies are also confirming that untreated hearing loss is associated with poor health outcomes including an increased risk of depression2, falls3-4, and earlier onset of cognitive decline5-6. One goal of OTC HAs is to provide better access to lower-cost hearing aids which might be pursued sooner by some individuals.
While OTC HAs may be a good first step in getting people situational hearing help earlier, when an individual needs a more individualized solution, or when the degree of hearing loss increases, an audiologist should be involved to create a comprehensive plan of care.
You may want to consider offering OTC HAs as part of your practice to connect the individual to your practice sooner rather than later. Alternatively, you may want to provide information on your website and the opportunity to be seen in your clinic if an individual needs support with an OTC HA that they’ve already purchased. Again, this is one way to connect individuals to your practice. You want to be identified as the source of trusted information in all things related to hearing health and hearing assistance.
References
1Kochkin S. MarkeTrak VIII: 25-year trends in the hearing health market. Hearing Rev 2009; 16 (11) 12-31
2Golub JS, Brewster KK, Brickman AM, et al. Association of Audiometric Age-Related Hearing Loss with Depressive Symptoms among Hispanic Individuals. JAMA Otolaryngol - Head Neck Surg. 2019. doi:10.1001/jamaoto.2018.3270
3Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012. doi:10.1001/archinternmed.2011.728
4Agmon, M., Lavie, L., Doumas, M. The association between hearing loss, postural control, and mobility in older adults: A systematic review. J Am Acad Audiol. 2017. doi:10.3766/jaaa.16044
5Ray J, Popli G, Fell G. Association of Cognition and Age-Related Hearing Impairment in the English Longitudinal Study of Ageing. JAMA Otolaryngol - Head Neck Surg. 2018. doi:10.1001/jamaoto.2018.1656
6Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing Loss and Cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011. doi:10.1037/a0024238
Hearing Testing Questions
It is clear from all current federal proposed rules that audiologists should be able to dispense OTC HAs. It is not clear whether given the other constraints on audiological practice imposed by states whether you, as an audiologist, will be required to provide a hearing test prior to dispensing OTC HAs. Be sure to verify your state regulations.
Currently, states use the term “hearing aids” in regulations without differentiating between OTC HAs and prescription hearing aids. Over time, we will see states updating their language and the regulations surrounding Audiology practices will be clearer. We may see separate requirements related to the provision of OTC HAs and Prescription Hearing Aids when an audiologist is involved.
Requirements for hearing testing when an audiologist provides an OTC HA are not clear at this time even though the final FDA rule is published. On the one hand, FDARA (FDA Reauthorization Act of 2017) is clear that a state or local government cannot require persons engaged in commercial activity involving OTC hearing aids to undertake special licensing solely on that basis.
On the other hand, persons who voluntarily identify as a licensed person (i.e., audiologists) are subject to corresponding state or local requirements. The caveat is that the FDA qualifies this statement with “including consumer protection requirements to the extent that the state or local requirements do not restrict or interfere with commercial activity involving OTC Hearing aids.”
The final rule makes it clear that requiring a hearing test is restrictive and interferes with the consumer’s access to OTC HAs. At the same time, state licensing laws currently do not differentiate types of hearing aids, they just use the term “hearing aids.” Audiologists will need to work with state regulators to interpret what requirements for hearing testing are in place specifically when an Audiologist is the person dispensing the OTC HA.
Most audiologists will want to provide a diagnostic hearing evaluation as part of their standard services because it follows best practices in terms of diagnostic audiology leading to an individualized hearing-care plan. This also is true when the hearing-care plan includes OTC HAs.
This approach also would avoid potential reputational harm if individuals who are not candidates for OTC HAs purchase from the practice The Academy will be reviewing and monitoring state laws and updating membership accordingly.
It has long been the stance of the Academy that diagnostic hearing evaluations are essential for good hearing health. The requirement for a hearing evaluation prior to the purchase of an OTC HA is not included in the FDA rule given that the purpose of moving to OTC HAs was to create a direct-to-consumer option with no professional services required.
The Academy will continue to advocate on behalf of the patients we serve and you, audiologists, to communicate to the FDA that having a hearing test is best practice, is not a barrier to obtaining hearing habilitation and is likely covered by most insurances (including Medicare). Additionally, the Academy is educating consumers about OTC HAs and the role an audiologist and a diagnostic hearing test have in their hearing health-care journey.
Lastly, the Academy is planning allied health-care education about our role in this process, including targeted education for pharmacists, primary-care providers, and geriatricians.
The Academy has included FAQs for consumers that emphasize the wisdom of having a hearing test conducted by an audiologist prior to making any hearing-health decisions and the link to Find an Audiologist Directory is provided throughout the consumer information.
Yes. A hearing test can be required if you routinely require a hearing test to create a plan of treatment regardless of whether that plan includes a prescriptive fitting or an OTC HA.
Considering the proposed FDA rule, you cannot require a hearing test specifically to dispense an OTC HA. An explanation to patients about the advantages of a hearing evaluation prior to any hearing aid fitting would be beneficial in this scenario.
Should a patient decide to forego a hearing evaluation, documentation in the chart should clearly state that a hearing evaluation was recommended and declined. Each audiologist will need to monitor state laws that may dictate the need for a hearing test when an OTC HA is dispensed by an audiologist. Currently, it is not clear where states stand on this issue.
Legal and Regulatory Issues
Working with OTC HAs does not require any type of license, registration, or specific training. The audiology assistant should be able to instruct patients in OTC HA use and care. Audiologists and audiology assistants will need to work with state regulators to interpret what requirements for hearing testing are in place specifically when an audiology assistant is the person dispensing the OTC HA. Be sure to check your state regulations as indicated.
The FDA rule indicates that individual state regulations will not create barriers for consumers wishing to pursue OTC HAs.
The new rule is focused on OTC HAs, not prescription hearing aids. With that said, any new rule can have unintended consequences. In essence, the FDA is creating rules (and removing restrictions) to make sure that consumers in every state have access to OTC HAs without restriction or interference from state laws.
In doing this, the FDA has removed the need for medical clearance as dictated by the FDA when an individual pursues hearing aids. By removing this rule completely to remove potential restrictions on OTC HAs, the FDA has deferred to the states to regulate the sale of prescription hearing aids.
If your state has rules in place that mandate medical clearance, these will now be in effect related to your ability to provide prescription hearing aids. What is not clear at this time for a licensed audiologist is how this state-level rule will impact your ability to provide OTC HAs.
On the one hand, the FDA rule makes it clear that hearing tests and other evaluations cannot be required to receive an OTC HA, just as compelling is an individual’s license that refers to “hearing aids” at this time and does not differentiate between types of hearing aids. Until states update their language to differentiate between type of hearing aids, audiologists will need guidance at the state level in terms of any restrictions that may be put on them with regard to provision of OTC HAs.
The Academy has reached out the FDA for further clarification related to the role of audiologists in dispensing OTC HAs and the issues presented by the final rule and audiology state licenses. In addition, the Academy will be reviewing and monitoring state license laws to advise members.
The FDA rule does not require manufacturers to allow returns of OTC devices, but does require the return policy to be included in the labeling appearing outside of the box. By nature of an individual’s audiology license, returns and/or trial periods may be mandated by state rules. The FDA did not consider a state law mandating that licensed audiologists offer a trial and return period to interfere with or restrict the provision of OTC HAs. In essence, purchasing OTC HAs from a licensed professional may provide additional consumer protections.
If you believe a manufacturer or distributor of OTC HA devices is not following the published rules and current laws, you can report this concern to the FDA.
Device Questions
This issue is not specifically addressed in the FDA rule. The rule does require that a description of how to pursue device repair be included in the instructions as well as an email address and mailing address for the manufacturer.
Since OTC HAs are not intended to require the involvement of a licensed hearing health-care professional, it is assumed that the consumer has read the information provided about the product to inform a decision to purchase.
OTC HAs present an opportunity for you, as audiologists, to be a resource for patients (or potential patients). Practices may consider placing information on their website that provides general guidance about OTC HAs and offer appointments to patients who have purchased OTC HAs and require assistance.
The FDA rule relies on an existing voluntary consensus standard for personal sound amplification products (ANSI/CTA 2051: Personal Sound Amplification Performance Criteria [2017]) for the OTC HA minimum requirements. Depending on the OTC HA, an audiologist should be thoughtful about how to connect the device to the hearing aid analyzer coupler to make these measurements.
Perhaps more relevant to patient care, any device that produces sound can be measured using real-ear probe microphone measurements on the patient. If the goal is to determine if the amplification is correct for the patient, the fitting can be approached similarly to that of a prescriptive hearing aid—convert HL thresholds to SPL thresholds using real-ear-to-coupler difference, generate evidence-based targets, and measure hearing aid output using real-ear aided response.
Various levels of speech are presented and output is measured to compare against targets. Although flexibility of settings of OTC devices is not known at this time and will vary, if the OTC HA is adjustable, the audiologist may be able to tune the device to better meet the patient’s hearing needs. Professional fees should be charged for these services.
This capability will depend on the manufacturer making and supplying the OTC HA.
OTC HAs are intended to be “customizable” and to not require the involvement of a hearing health-care professional. At a minimum, the FDA is requiring that OTC HAs have a way to adjust frequency response (e.g., bass versus treble) and volume. A “self-fitting” device is a type of customizable device that makes changes to the programming based on user response to some type of signal (e.g., online hearing test).
More Information and Support
Yes, the Academy will continue to provide and update information for members, allied health-care professionals, and for consumers. See the Academy website for current information relative to OTC HAs.
Be sure to visit the Academy’s Consumer website. There you can review FAQs for Consumers related to OTC HAs as well as guidance to ensure parents recognize that OTC HAs are not for children.
The Academy’s e-learning platform, www.eaudiology.org, currently has five OTC hearing aid web seminars available on demand and will be adding another session delivered at the AAA 2022+HearTECH Expo. Look forward to additional webinars and educational offerings focused on OTC hearing aids once the final FDA rule is released.
Register for our upcoming OTC hearing aid web seminar on November 1 here.
Clinicians can consider the following when communicating the need to see an audiologist as part of a productive rehabilitative plan for hearing loss. A hearing evaluation can help a consumer be confident that they are a candidate. You may want to promote your diagnostic services to assure individuals that they can receive an assessment and advice without purchasing a solution directly from your practice. Or you may want to let the public know that you will provide a plan of treatment that will include OTC Has if they are appropriate for the individual. Alternatively, you may focus your efforts on individuals who are looking for customized solutions.
Language describing what the audiologist offers in terms of customization is provided below and you may find this helpful in communicating with patients asking about “why they should see an audiologist.”
- The most common hearing loss in adults comes on gradually. Individuals often do not identify the level of their hearing loss accurately because of these gradual changes over time. This makes it difficult for individuals to identify accurately if they are a candidate for over-the-counter hearing aids (someone with a mild to moderate hearing loss).
- When you come to an audiologist thinking about getting hearing aids, the audiologist uses the information from the hearing test as well as information from an in-depth interview with you about your lifestyle, listening needs, and communication demands.
- It may seem like picking a hearing aid is straightforward, but quite a bit of information must be collected to make the best choice for you.
- The degree of hearing loss, ear-canal characteristics, and individual preferences will all impact the recommendation of style (what the hearing aid physically looks like). Your lifestyle and communication demands impact the technology and features that should be selected and how these are programmed in the hearing aid fitting.
- Because of the gradual nature of the onset of adult hearing loss, your brain is tuned to listening through the hearing loss and considers that input “normal” even though sound is being filtered by the hearing loss. Because the brain thinks that is normal, it is difficult for you to judge how much amplification is needed when you try hearing aids.
- People typically have different amounts of hearing loss at different pitches which complicates this even more. ome people may hear low-pitch sounds fine, but cannot hear or understand high-pitched sounds. When an audiologist fits a hearing aid, they put a microphone in your ear canal to measure the output of the hearing aid at different presentation levels across different pitches to tune the hearing aid specifically to your individual ear canal and hearing loss. This ensures that sounds are at the correct level to be heard but not so loud that they can injure your hearing.
- On the first day of the hearing aid fitting, most patients indicate that sounds don’t seem normal to them and that is because the brain is not used to hearing many of these softer sounds. It may take several weeks of full-time use (all waking hours) to adjust to a new hearing aid fitting because the brain must adapt to this new normal. Without these measurements, it is impossible to know if the amplification device has been set optimally for you.
- If you pursue an OTC HA and do not feel that you are receiving the benefit you expected, you may want to schedule with an audiologist who can make these measurements in the ear canal and provide you with advice about how you might want to re-set the device if it has controls for fine-tuning. You would expect to pay an office visit fee for these services.
- Remember that if you are exposed to loud sound over time, your hearing can be damaged. If you are concerned that your hearing has changed, you will want to see an Audiologist for an updated hearing test.”
While hearing aids are intended to treat impaired hearing, PSAPs are meant for individuals with normal hearing who want to amplify sounds in certain situations, for example, bird watching. PSAPs are regulated as consumer electronics as opposed to medical devices so quality can vary widely. The FDA does not regulate PSAPs for safety and effectiveness like it does hearing aids. Patients should be careful not to keep the device on if the sound is very loud, since hearing could be damaged over time. PSAPs are usually low cost (less than $100.00) and simple to operate.
Hearables are worn in or on the user’s ears (e.g., headset, earbuds) and wirelessly receive a sound signal from another device (e.g., phone or TV). The headset may have a volume control or an app associated with it so the user can turn the volume up or down to meet their listening needs. Hearables provide situation-specific listening solutions (e.g., phone, television) rather than a hearing solution for a listener’s entire day of communication. Some hearables allow the user to adjust the pitch or loudness of the sound. These devices also may provide biometric tracking (how many steps walked, heartrate, etc.). This type of wireless connectivity can be included in professionally fit hearing aids as well, but the hearing aids are also providing custom amplification to the signals being heard and are used throughout the day.
Hearables are not regulated by the FDA for safety and effectiveness. The user will want to be thoughtful about how loud they listen to sound. If a user is concerned about the level of sound they are exposed to, an audiologist can measure the output of the headset and let the user know if they are in a safe listening range depending on how much time is spent listening at that level.
Interestingly, the FDA has not chosen to classify a device (Hearable) that adapts the output of other hearing products, such as earbuds, to compensate for perceived mild to moderate hearing impairment.
Make sure people can find you and your practice as they start to navigate hearing health care by having up-to-date contact information listed on the Find an Audiologist Directory.
Want to take this information for your personal use or to share with others? Access a PDF copy of the FAQs below.